This may sound a little confusing as the monitoring system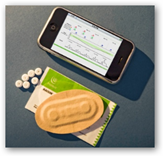 and the smart pill received separate approvals from the FDA. There’s also a smart patch which reports back to the Proteus system and that part has been approved. See who the big investors are here with the product, none other that Novartis and Medtronic. I remember it was reported that Novartis has $24 million invested so I am not sure if there are others and the amount contributed by Medtronic.
and the smart pill received separate approvals from the FDA. There’s also a smart patch which reports back to the Proteus system and that part has been approved. See who the big investors are here with the product, none other that Novartis and Medtronic. I remember it was reported that Novartis has $24 million invested so I am not sure if there are others and the amount contributed by Medtronic.
FDA Clearance of Wireless Proteus Wireless Monitoring Device Reported – Novartis and Medtronic Have $50 Million Invested in the “Raisin”
Now the European CE was a little ahead with the pill portion  of this and gave their approval back in August of 2010. So now the chip can send even more information and is made out of minerals found in food, such as copper, magnesium and silicon. There’s no battery on the chip and we kind of all can figure out where the exit point is when done. As I read this, the minute the pill hits your stomach, it gets powered for action right there when it gets wet. The chip is placed on your pills you take for prescriptions. I would guess that drugs made by Novartis could be the first ones to be in line to carry the chip? Here’s a video of a company representative explaining how this works and you can “share” with other folks who have similar conditions. I don’t know if I’m ready to share what goes on in my stomach and bowels with others yet, maybe more information than some may want to know. BD
of this and gave their approval back in August of 2010. So now the chip can send even more information and is made out of minerals found in food, such as copper, magnesium and silicon. There’s no battery on the chip and we kind of all can figure out where the exit point is when done. As I read this, the minute the pill hits your stomach, it gets powered for action right there when it gets wet. The chip is placed on your pills you take for prescriptions. I would guess that drugs made by Novartis could be the first ones to be in line to carry the chip? Here’s a video of a company representative explaining how this works and you can “share” with other folks who have similar conditions. I don’t know if I’m ready to share what goes on in my stomach and bowels with others yet, maybe more information than some may want to know. BD
Proteus Digital Health Inc. (Redwood City, CA) has announced that FDA has cleared its swallow-able sensor, which can be integrated into a pill to  monitor medication adherence. The de novo medical device, which the company refers to as an ingestible event marker (IEM), can monitor the timing of drug ingestion in the stomach and relay that information to a patch worn on the body. The patch also can track heart rate, activity levels, and sleep patterns, and relay such information, as well as information regarding drug compliance to caregivers via text message or e-mail. In addition, the device can be used to send text reminders to patients who forgot to take a pill on time.
monitor medication adherence. The de novo medical device, which the company refers to as an ingestible event marker (IEM), can monitor the timing of drug ingestion in the stomach and relay that information to a patch worn on the body. The patch also can track heart rate, activity levels, and sleep patterns, and relay such information, as well as information regarding drug compliance to caregivers via text message or e-mail. In addition, the device can be used to send text reminders to patients who forgot to take a pill on time.
http://www.mddionline.com/article/fda-gives-510k-nod-ingestible-smart-pill



0 comments :
Post a Comment